cfHPV-DNA represents a promising surrogate of disease burden in patients with HPV-driven cancers.
Applying ultra-sensitive technology to detect and quantify circulating HPV-DNA using HPV-SEQ is an innovative and exciting step forward in the development of more targeted approaches when managing HPV-associated cancers.
HPV-SEQ has high analytical and clinical sensitivity, with the ability to reliably and consistently detect as low as 2 copies of HPV 16 and HPV 18 DNA.
Download info on our latest clinical trials
HPV-SEQ achieves ultra-sensitive detection and accurate quantification of HPV 16 and HPV 18 through a unique, proprietary method to better detect clinically meaningful changes.
Clinical sensitivity of detecting cfHPV-DNA in pretreatment baseline using HPV-SEQ was 97.6% (95% CI, 91.5% – 99.7% [80 of 82 tests])
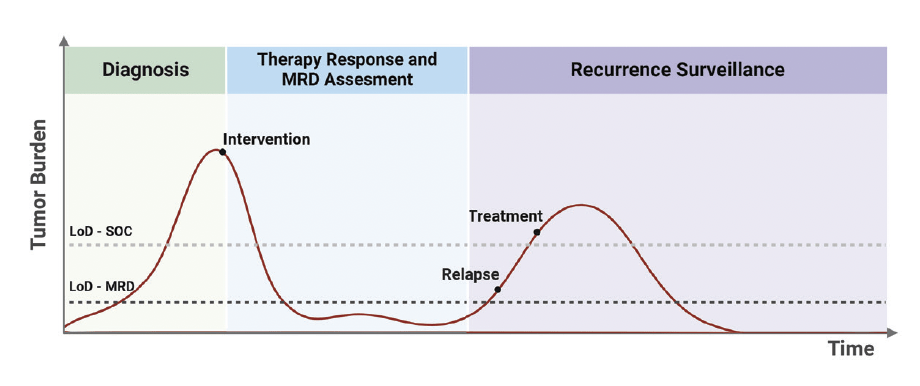
cfHPV-DNA is a sensitive, dynamic and non-invasive biomarker that has clinical utility in various stages of HPV-related cancer journey.
- cfHPV-DNA may be used for assessing patients’ trial eligibility, by determining patients’ HPV status and subtype.
- cfHPV-DNA may be used as a surrogate for real-time monitoring of treatment response.
- Longitudinal cfHPV-DNA may be used to monitor disease recurrence in post-treatment surveillance setting, after curative intent therapy.
- Ultra-sensitive detection of cfHPV-DNA may enable more informed therapeutic approaches for management of HPV-driven cancers.
- cfHPV-DNA monitoring may be used to grade treatment responses to select patients for treatment de-intensification (currently under investigation in ongoing trials).
cfHPV-DNA panel
Test name | Subtypes detected | Clinically intended uses | Sample types accepted | Sample requirements | Turnaround time |
|---|---|---|---|---|---|
HPV-SEQ | HPV 16, HPV 18 |
|
|
| 7 – 10 Business days |
Media
Advancing care & management of patients with HPV driven head & neck cancers
Watch Dr. Nishant Agrawal as he discusses head and neck cancers, the role played by the Human papillomavirus (HPV), and an ongoing study to personalize and improve treatment of patients using quantitative cell-free HPV-DNA (cfHPV-DNA) in plasma as a biomarker for grading treatment response.
cfHPV-DNA: A new prognostic biomarker in HPV driven OPSCC
Watch as the speakers explore the role cfHPV-DNA plays in cancer prognosis, and what barriers biopharma companies face in incorporating cfHPV-DNA into their clinical trial design. The speakers discuss how pharma manufacturers, HCPs and patients can collaborate to help overcome these barriers.